Experiments with salts
Refresh your memory!
If the words dissolution, solute, solvent, and dissolving don't ring a bell anymore, your can check the basics at Mixtures. Then, open the Concentration simulation (below on handheld devices, on the right otherwise). It works as follows:
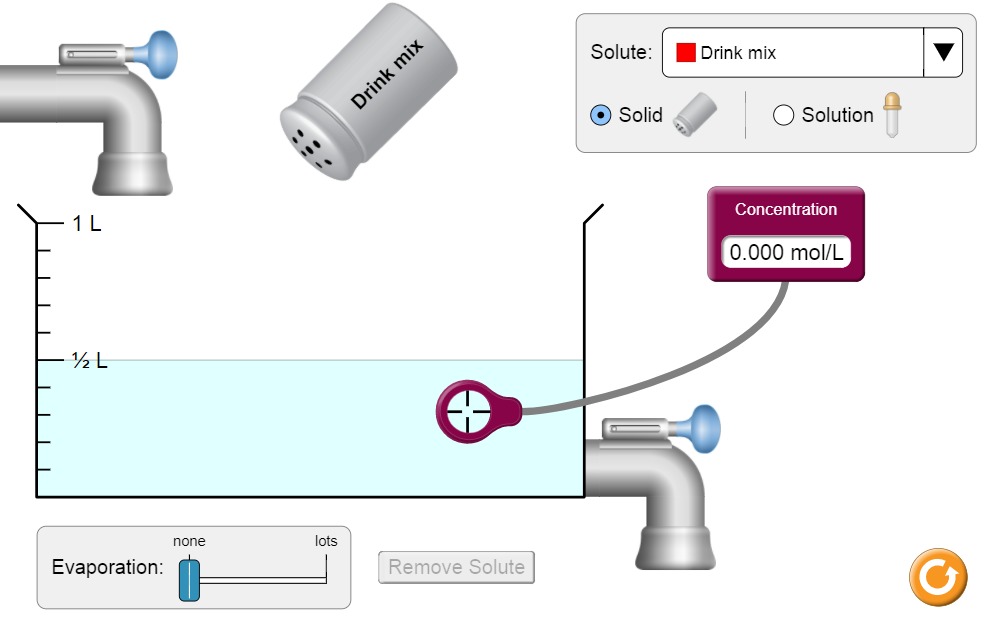
- If you make a mistake, you can reset the simulation by pressing the orange button on the right.
- First, you need to choose the substance to add to water from the Solute dropdown menu. The default is Drink mix.
- Then, place the purple concentration gauge into the solution. In the beginning, it should show 0.000 mol/L.
