Acid-base reactions
Measuring acidity
Equipment:
- 2x 100 ml beaker
- 50 ml of water in each one
- 3 drops of BTB in each one
- 1x straw
- 1 bottle of [[$\text{HCl(aq)}$]]
- 1 bottle of [[$\text{NaOH(aq)}$]]
- 10 slips of pH paper
- 1 tissue
- Pick either beaker.
- Blow air into the liquid with a straw.
- Caution! Do not inhale, just exhale.
- Caution! Blow gently to prevent spills.
- Observe the change.
- Write the reaction as an equation.
- What is the substance you exhale?
- What is the substance you exhale into?
- What is the substance the reaction yields?
- Switch to the unused beaker
- Add 15 drops of [[$\text{HCl}$]] into the solution, one drop at a time.
- Measure the pH of the solution with a slip of pH paper.
- Place the slip on the tissue.
- Add 3 drops of [[$\text{NaOH}$]] into the solution.
- Repeat from step 7 onwards until the pH slips are exhausted.
- Plot the pH changes as a diagram.
- horizontal: drops of [[$\text{NaOH}$]]
- vertical: pH of solution
- With a trendline, find the amount of [[$\text{NaOH}$]] drops needed for pH 7.
Measuring buffering ability
Equipment:
- 100 ml beaker
- 20 ml of milk
- 3 drops of BTB
- 1 bottle of [[$\text{HCl(aq)}$]]
- 1 bottle of [[$\text{NaOH(aq)}$]]
- 10 slips of pH paper
- 1 tissue
- Add 15 drops of [[$\text{HCl}$]] into the solution, one drop at a time.
- Measure the pH of the solution with a slip of pH paper.
- Place the slip on the tissue.
- Add 3 drops of [[$\text{NaOH}$]] into the solution.
- Repeat from step 1 onwards until the pH slips are exhausted.
- Plot the pH changes as a diagram.
- horizontal: drops of [[$\text{NaOH}$]]
- vertical: pH of solution
- With a trendline, find the amount of [[$\text{NaOH}$]] drops needed for pH 7.
Examples
Example. When mixed with water, hydrochloric acid [[$\text{HCl}$]] dissociates into hydrogen ions [[$\text{H}^+$]] and chloride ions [[$\text{Cl}^-$]]:
[[$$\text{HCl} \rightarrow \text{H}^+ + \text{Cl}^-$$]]
In this context, water acts as a base for the hydrochloric acid. Water molecules [[$\text{H}_2\text{O}$]] absorb the dissociated hydrogen ions [[$\text{H}^+$]] to yield hydronium ions [[$\text{H}_3\text{O}^+$]]:
[[$$\text{HCl} + \text{H}_2\text{O} \rightarrow \text{H}_3\text{O}^+ + \text{Cl}^-$$]]
Example.
When mixing water solutions of hydrochloric acid [[$\text{HCl}$]] and sodium hydroxide [[$\text{NaOH}$]], both ionic compounds dissociate into positive and negative ions:
[[$$\text{HCl} + \text{NaOH} \rightarrow \text{H}^+ + \text{Cl}^- + \text{Na}^+ +\text{OH}^-$$]]
In this context, the hydroxide in sodium hydroxide acts as the base for the hydrochloric acid. Each hydrochloric acid particle donates a hydrogen ion [[$\text{H}^+$]] to one sodium hydroxide particle. In simpler words, the acidic [[$\text{H}^+$]] and the basic [[$\text{OH}^-$]] combine to make water:
[[$$\text{H}^+ + \text{Cl}^- + \text{Na}^+ + \text{OH}^- \rightarrow \text{NaCl} + \text{H}_2\text{O}$$]]
As ions of opposite charge, the remaining sodium ions [[$\text{Na}^+$]] and chloride ions [[$\text{Cl}^-$]] associate to form sodium chloride [[$\text{NaCl}$]].
[[$$\text{HCl} \rightarrow \text{H}^+ + \text{Cl}^-$$]]
In this context, water acts as a base for the hydrochloric acid. Water molecules [[$\text{H}_2\text{O}$]] absorb the dissociated hydrogen ions [[$\text{H}^+$]] to yield hydronium ions [[$\text{H}_3\text{O}^+$]]:
[[$$\text{HCl} + \text{H}_2\text{O} \rightarrow \text{H}_3\text{O}^+ + \text{Cl}^-$$]]
Example.
When mixing water solutions of hydrochloric acid [[$\text{HCl}$]] and sodium hydroxide [[$\text{NaOH}$]], both ionic compounds dissociate into positive and negative ions:
[[$$\text{HCl} + \text{NaOH} \rightarrow \text{H}^+ + \text{Cl}^- + \text{Na}^+ +\text{OH}^-$$]]
In this context, the hydroxide in sodium hydroxide acts as the base for the hydrochloric acid. Each hydrochloric acid particle donates a hydrogen ion [[$\text{H}^+$]] to one sodium hydroxide particle. In simpler words, the acidic [[$\text{H}^+$]] and the basic [[$\text{OH}^-$]] combine to make water:
[[$$\text{H}^+ + \text{Cl}^- + \text{Na}^+ + \text{OH}^- \rightarrow \text{NaCl} + \text{H}_2\text{O}$$]]
As ions of opposite charge, the remaining sodium ions [[$\text{Na}^+$]] and chloride ions [[$\text{Cl}^-$]] associate to form sodium chloride [[$\text{NaCl}$]].
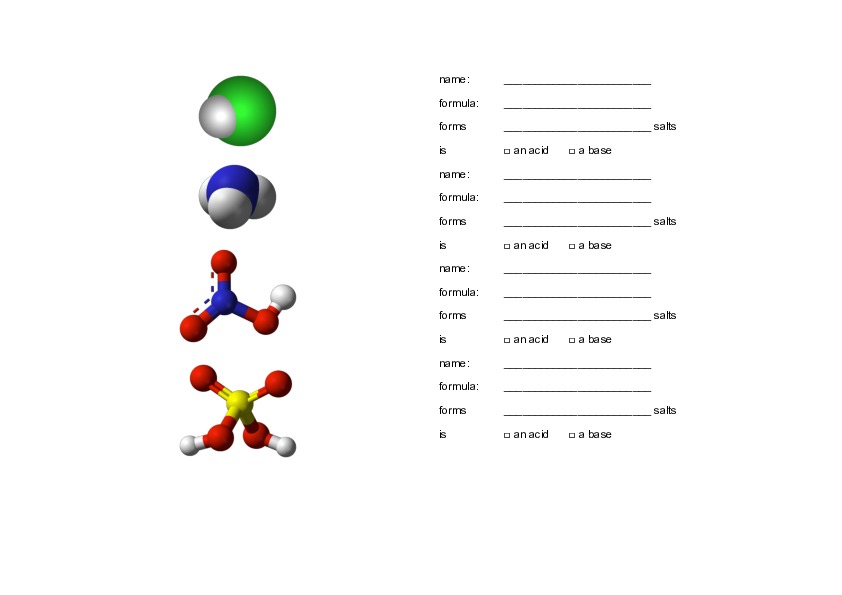
Acids and their salts
| hydrochloric acid | [[$\text{HCl}$]] | -chloride |
| sulphuric acid | [[$\text{H}_2\text{SO}_4$]] | -sulphate |
| nitric acid | [[$\text{HNO}_3$]] | -nitrate |
| carbonic acid | [[$\text{H}_2\text{CO}_3$]] | -carbonate |
| phosphoric acid | [[$\text{H}_3\text{PO}_4$]] | -phosphate |
Example.
a. hydrochloric acid + lithium hydroxide [[$\rightarrow$]] lithium chloride + water
[[$$\text{HCl} + \text{LiOH} \rightarrow \text{LiCl} + \text{H}_2$$]]
b. sulphuric acid + calcium hydroxide [[$\rightarrow$]] calcium sulphate + water
[[$$\text{H}_2\text{SO}_4 + \text{Ca(OH)}_2 \rightarrow \text{CaSO}_4 + 2\text{H}_2\text{O}$$]]