Forming molecular compounds
From covalent bonds into molecular compounds
Using the logic behind the periodic table studied in detail in From bonds to reactions,
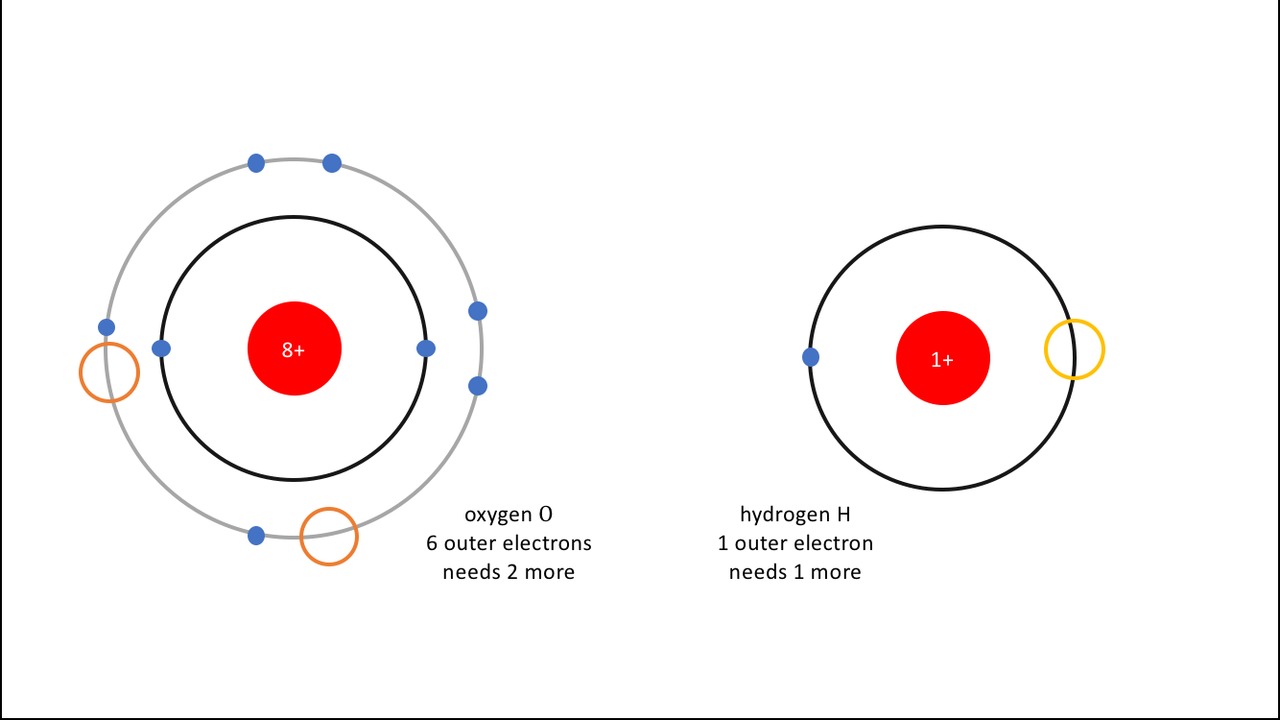
In the following figure, these missing electrons are marked as orange in the oxygen atom and as yellow in the hydrogen atom. It is clearly visible that a single oxygen atom needs two hydrogen atoms to satisfy its octet needs.
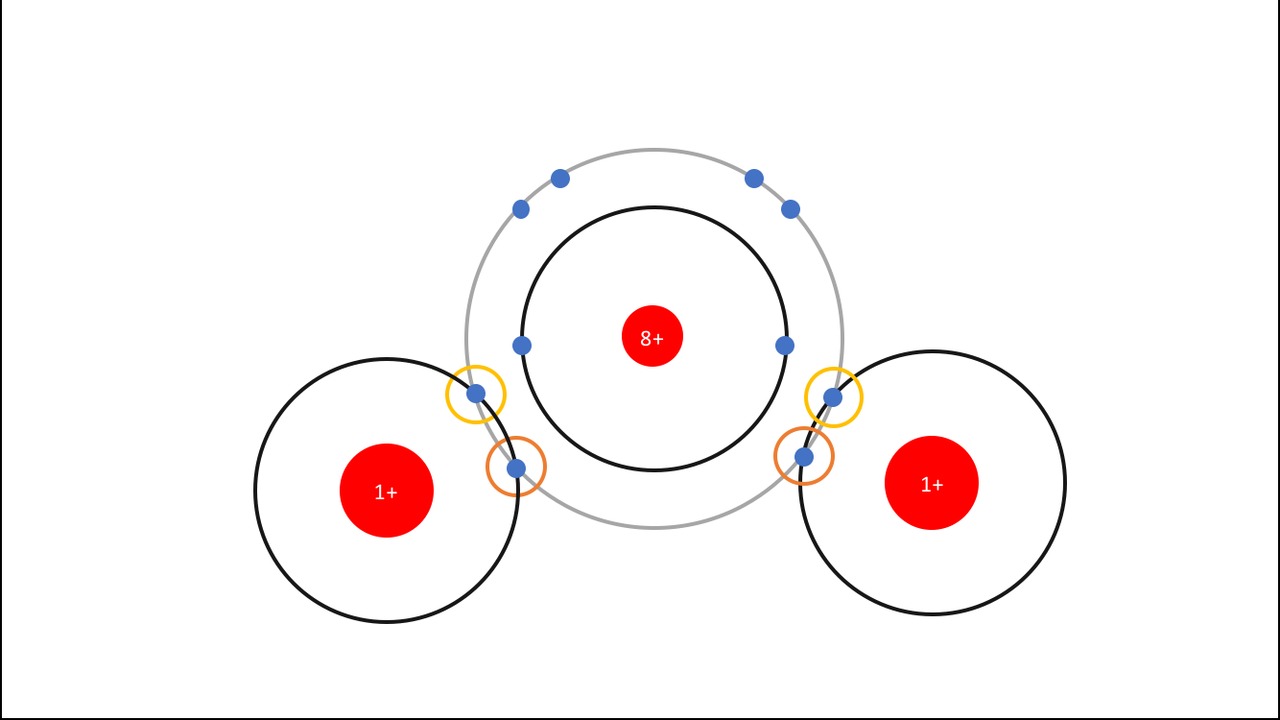
 This hydrogen-oxygen reaction produces dihydrogen monoxide [[$\text{H}_2\text{O}$]] also known as water.
This hydrogen-oxygen reaction produces dihydrogen monoxide [[$\text{H}_2\text{O}$]] also known as water.
 Both hydrogen and oxygen are found as gases [[$\text{H}_2$]] and [[$\text{O}_2$]] under normal temperature and pressure. This means that we need two hydrogen molecules for each oxygen molecules, as well. Symbolically:
Both hydrogen and oxygen are found as gases [[$\text{H}_2$]] and [[$\text{O}_2$]] under normal temperature and pressure. This means that we need two hydrogen molecules for each oxygen molecules, as well. Symbolically:
[[$$\underbrace{2\text{H}_2}_{\text{two hydrogen molecules}}+\underbrace{\text{O}_2}_{\text{one oxygen molecule}}\rightarrow \underbrace{2\text{H}_2\text{O}}_{\text{two water molecules}}$$]]
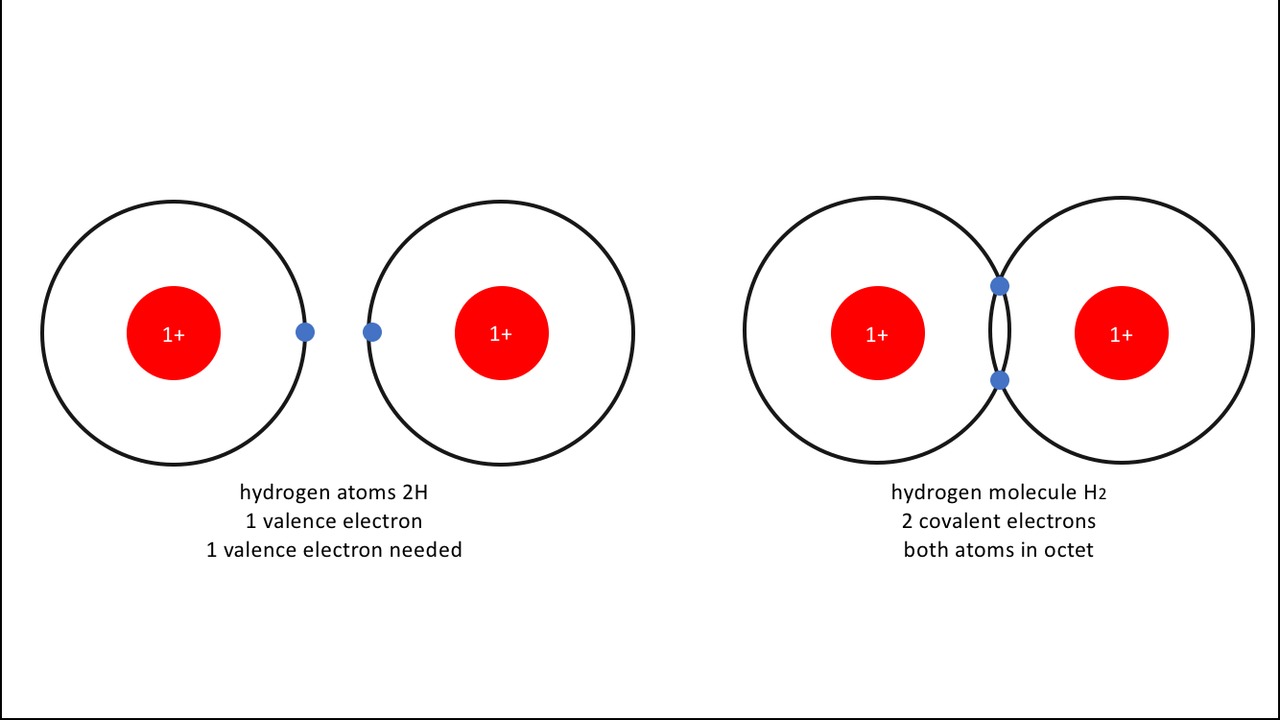
The electrons on the outest shell of an atom are called valence electrons, and for this reason, the bond holding a water molecule together is called a covalent bond. A molecule is a bundle of atoms bound to each other by covalent shared electrons.
A covalent bond is also the difference between having two hydrogen atoms [[$2\text{H}$]] and a two-atom hydrogen molecule [[$\text{H}_2$]]. Having two hydrogen atoms form a molecule is energetically more favourable than having them as separate atoms. This same principle applies to most non-noble gases, like oxygen [[$\text{O}_2$]] and nitrogen [[$\text{N}_2$]].
- H, hydrogen (vety) gains the octet state by receiving one additional electron, and
- O, oxygen (happi) gains the octet state by receiving two additional electrons.
In the following figure, these missing electrons are marked as orange in the oxygen atom and as yellow in the hydrogen atom. It is clearly visible that a single oxygen atom needs two hydrogen atoms to satisfy its octet needs.
 This hydrogen-oxygen reaction produces dihydrogen monoxide [[$\text{H}_2\text{O}$]] also known as water.
This hydrogen-oxygen reaction produces dihydrogen monoxide [[$\text{H}_2\text{O}$]] also known as water. Both hydrogen and oxygen are found as gases [[$\text{H}_2$]] and [[$\text{O}_2$]] under normal temperature and pressure. This means that we need two hydrogen molecules for each oxygen molecules, as well. Symbolically:
Both hydrogen and oxygen are found as gases [[$\text{H}_2$]] and [[$\text{O}_2$]] under normal temperature and pressure. This means that we need two hydrogen molecules for each oxygen molecules, as well. Symbolically:[[$$\underbrace{2\text{H}_2}_{\text{two hydrogen molecules}}+\underbrace{\text{O}_2}_{\text{one oxygen molecule}}\rightarrow \underbrace{2\text{H}_2\text{O}}_{\text{two water molecules}}$$]]
The electrons on the outest shell of an atom are called valence electrons, and for this reason, the bond holding a water molecule together is called a covalent bond. A molecule is a bundle of atoms bound to each other by covalent shared electrons.
A covalent bond is also the difference between having two hydrogen atoms [[$2\text{H}$]] and a two-atom hydrogen molecule [[$\text{H}_2$]]. Having two hydrogen atoms form a molecule is energetically more favourable than having them as separate atoms. This same principle applies to most non-noble gases, like oxygen [[$\text{O}_2$]] and nitrogen [[$\text{N}_2$]].
