Forming ionic compounds
From ionic bonds into ionic compounds
Using the logic behind the periodic table studied in detail in From bonds to reactions,
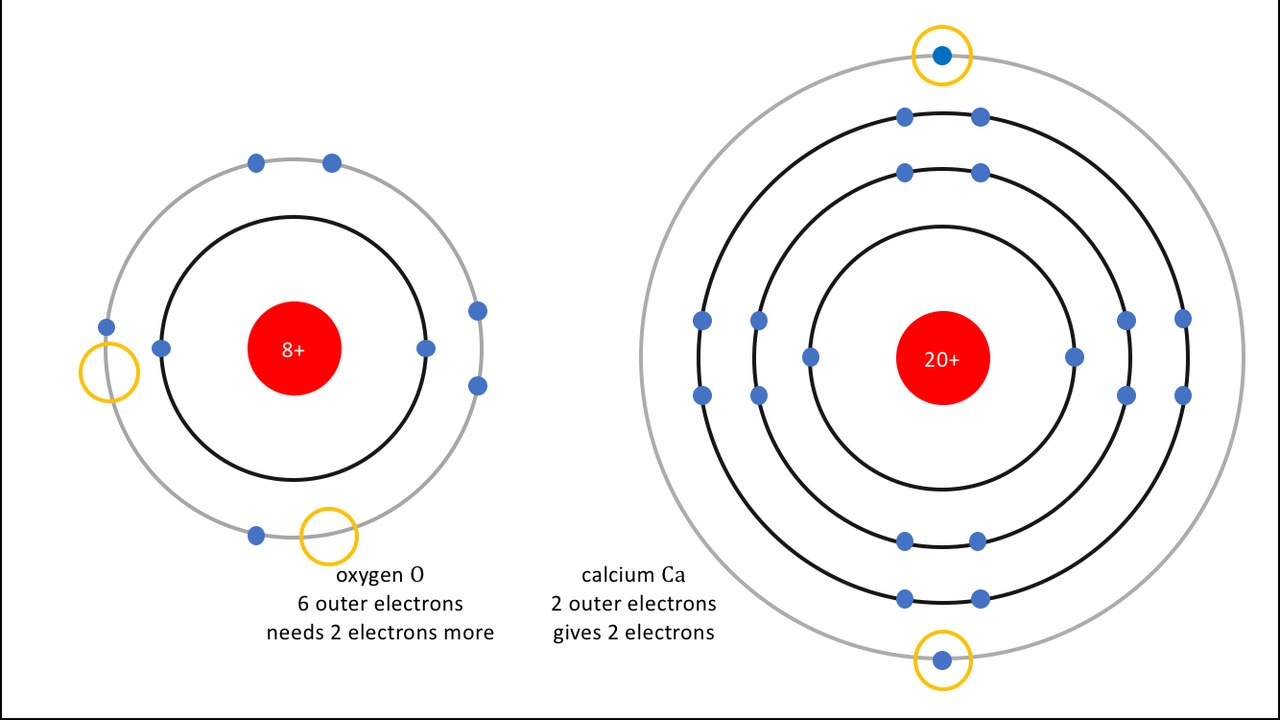
Symbolically, this flame reaction can be written simply as:
[[$$ \text{Ca}+\text{O}\rightarrow \text{CaO} $$]]
However, as oxygen is never found as plain molecules [[$ \text{O} $]] but as double-atom molecules [[$ \text{O}_2 $]], this equation must be balanced. You cannot create, you cannot destroy any atoms, so the amounts of oxygen and calcium must match on both sides of this reaction:
[[$$ \underbrace{2\text{Ca}}_{\text{two calcium atoms}}+\underbrace{\text{O}_2}_{\text{oxygen molecule}}\rightarrow \underbrace{2\text{CaO}}_{\text{two units of calcium oxide}} $$]]
This means that for each oxygen molecule [[$ \text{O}_2 $]], we need two calcium atoms [[$ \text{Ca} $]] to get two units of calcium oxide [[$ \text{CaO} $]].
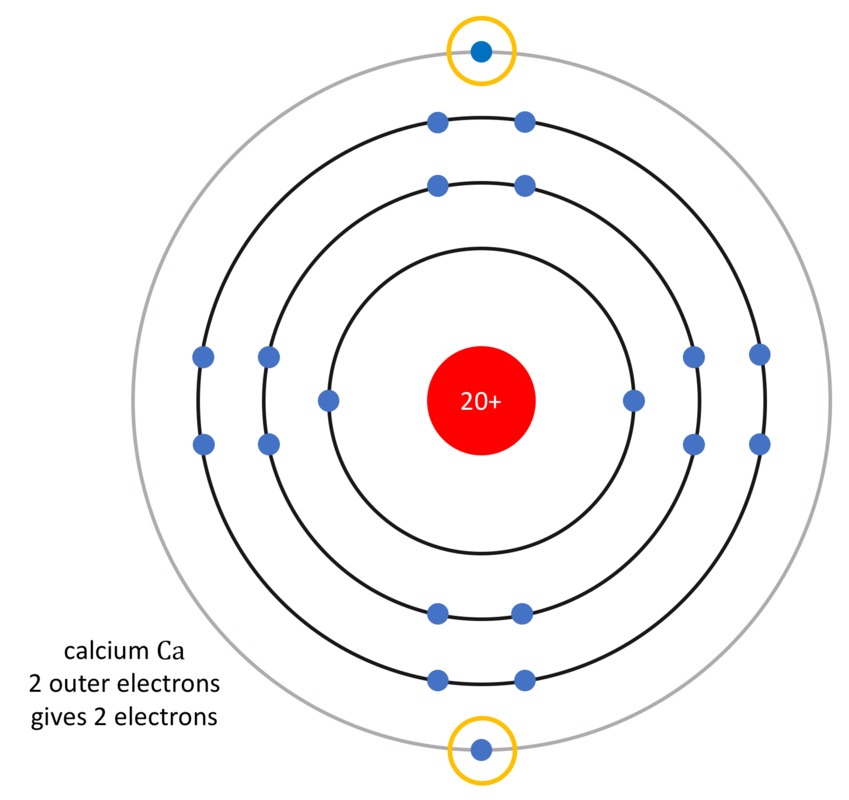
Let us take this one step at a time. When the reaction begins, the calcium atom [[$\text{Ca}$]] donates two electrons. As the electrons are negatively charged, the remaining part of the calcium atom must be of positive charge. This creates a calcium ion [[$\text{Ca}^{2+}$]]. Symbolically,
[[$$ \underbrace{\text{Ca}}_{\text{calcium atom}} \rightarrow \underbrace{\text{Ca}^{2+}}_{\text{calcium ion}} + \underbrace{2e^{-}}_{\text{two electrons}} $$]]
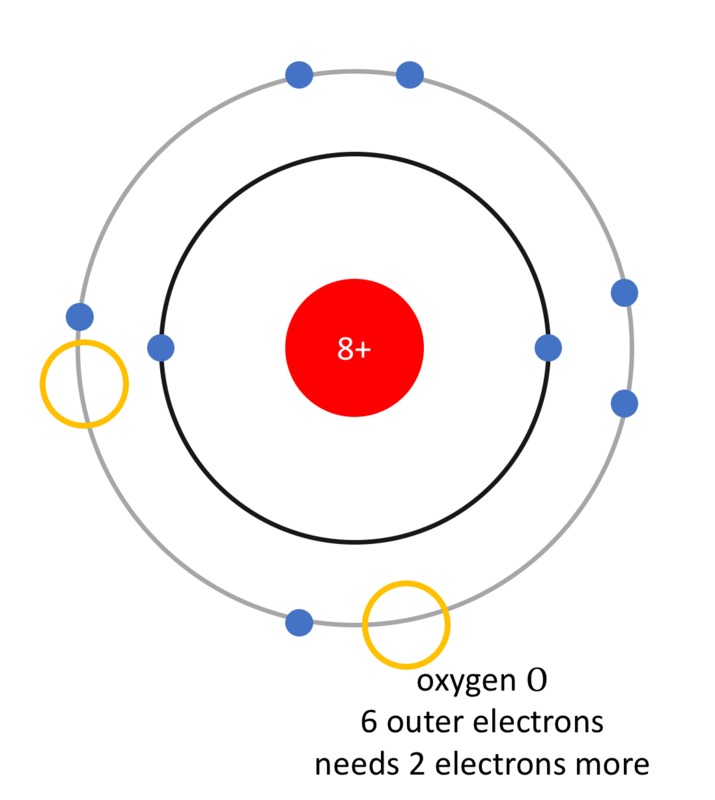
Likewise, when the oxygen atoms [[$\text{O}$]] in [[$\text{O}_2$]] receive electrons, they form oxygen ions called oxide ions [[$\text{O}^{2-}$]] of opposite negative charge. Symbolically,
[[$$ \underbrace{\text{O}}_{\text{oxygen atom}} + \underbrace{2e^{-}}_{\text{two electrons}} \rightarrow \underbrace{\text{O}^{2-}}_{\text{oxide ion}}$$]]
The charges in the calcium ion [[$\text{Ca}^{2+}$]] and the oxide ion [[$\text{O}^{2-}$]] match. The opposite charges attract each other, as dictated by one of the Fundamental interactions. Symbolically, we obtain calcium oxide:
[[$$\underbrace{\text{Ca}^{2+}}_{\text{calcium ion}} + \underbrace{\text{O}^{2-}}_{\text{oxide ion}} \rightarrow \underbrace{\text{CaO}}_{\text{calcium oxide}}$$]]
- Ca, calcium (kalsium) gains the octet state by donating two electrons, and
- O, oxygen (happi) gains the octet state by receiving two electrons.

Symbolically, this flame reaction can be written simply as:
[[$$ \text{Ca}+\text{O}\rightarrow \text{CaO} $$]]
However, as oxygen is never found as plain molecules [[$ \text{O} $]] but as double-atom molecules [[$ \text{O}_2 $]], this equation must be balanced. You cannot create, you cannot destroy any atoms, so the amounts of oxygen and calcium must match on both sides of this reaction:
[[$$ \underbrace{2\text{Ca}}_{\text{two calcium atoms}}+\underbrace{\text{O}_2}_{\text{oxygen molecule}}\rightarrow \underbrace{2\text{CaO}}_{\text{two units of calcium oxide}} $$]]
This means that for each oxygen molecule [[$ \text{O}_2 $]], we need two calcium atoms [[$ \text{Ca} $]] to get two units of calcium oxide [[$ \text{CaO} $]].
Let us take this one step at a time. When the reaction begins, the calcium atom [[$\text{Ca}$]] donates two electrons. As the electrons are negatively charged, the remaining part of the calcium atom must be of positive charge. This creates a calcium ion [[$\text{Ca}^{2+}$]]. Symbolically,
[[$$ \underbrace{\text{Ca}}_{\text{calcium atom}} \rightarrow \underbrace{\text{Ca}^{2+}}_{\text{calcium ion}} + \underbrace{2e^{-}}_{\text{two electrons}} $$]]

Likewise, when the oxygen atoms [[$\text{O}$]] in [[$\text{O}_2$]] receive electrons, they form oxygen ions called oxide ions [[$\text{O}^{2-}$]] of opposite negative charge. Symbolically,
[[$$ \underbrace{\text{O}}_{\text{oxygen atom}} + \underbrace{2e^{-}}_{\text{two electrons}} \rightarrow \underbrace{\text{O}^{2-}}_{\text{oxide ion}}$$]]

The charges in the calcium ion [[$\text{Ca}^{2+}$]] and the oxide ion [[$\text{O}^{2-}$]] match. The opposite charges attract each other, as dictated by one of the Fundamental interactions. Symbolically, we obtain calcium oxide:
[[$$\underbrace{\text{Ca}^{2+}}_{\text{calcium ion}} + \underbrace{\text{O}^{2-}}_{\text{oxide ion}} \rightarrow \underbrace{\text{CaO}}_{\text{calcium oxide}}$$]]